Technology
Our Approach & Technology
The Molecular Clamp technology
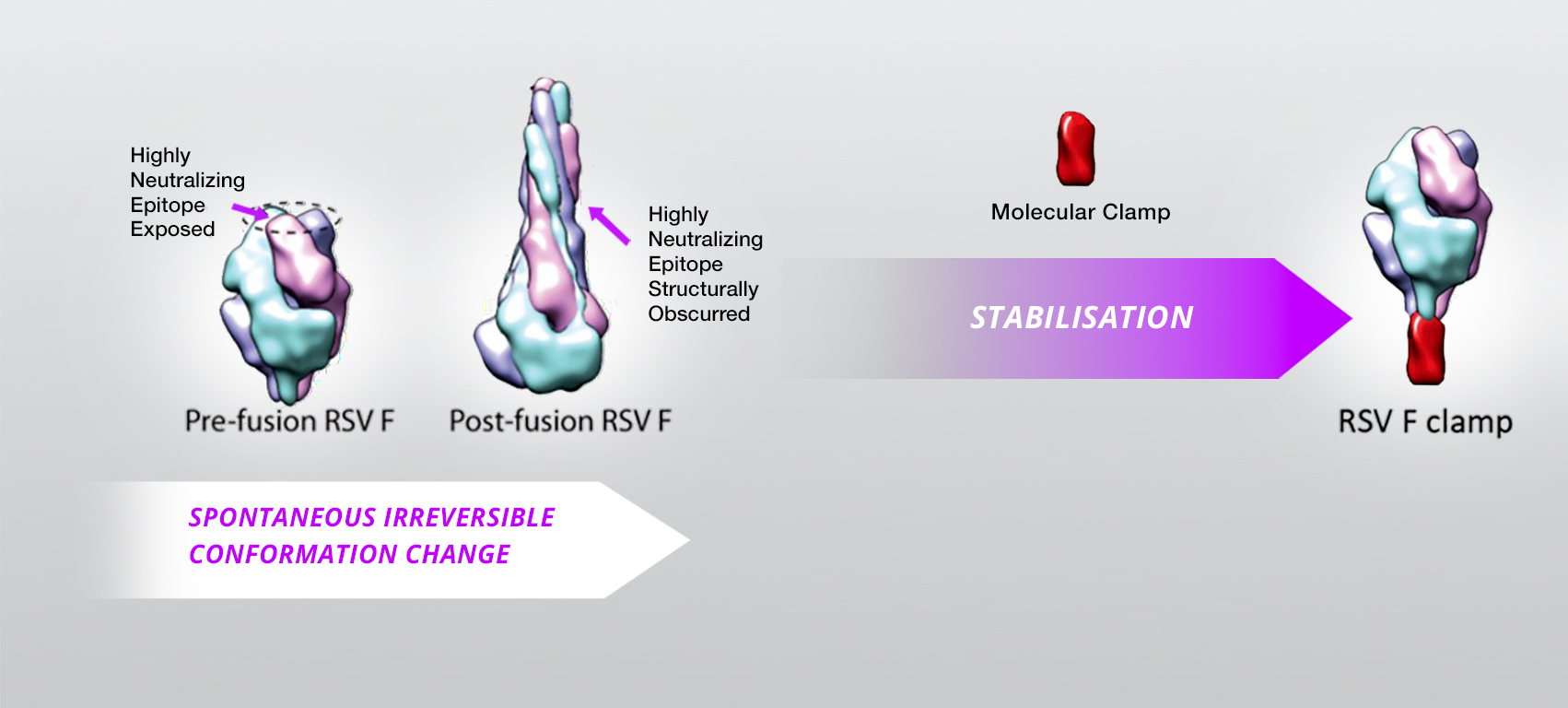
The key technical advantage of the Molecular Clamp is its ability to impart superior stability over alternate trimerisation approaches, which enables consistent and high yield production of viral fusion proteins in their native trimeric “prefusion” form.
A vaccine’s efficacy relies on its ability to stimulate the immune system with antigens in the correct conformation. Producing these proteins in such conformation, in a form that allows long term storage and convenient administration remains a major challenge.
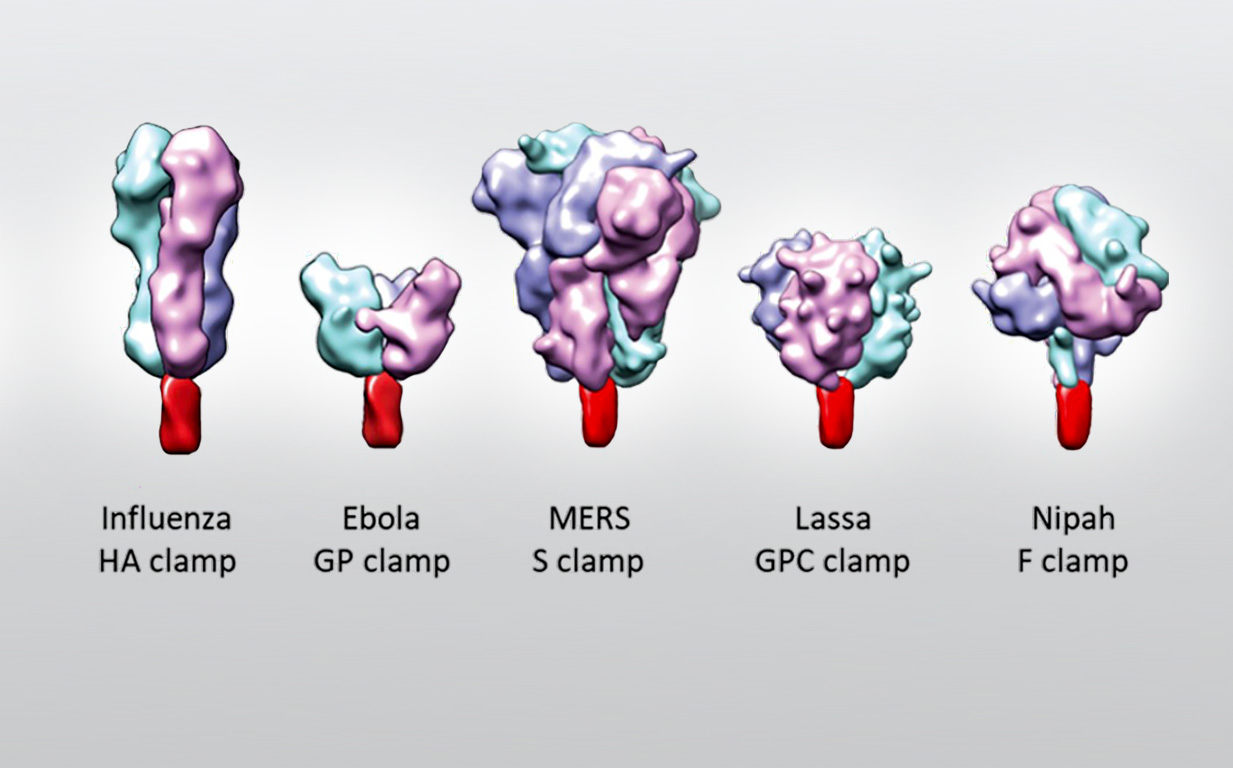
The Molecular Clamp technology is able to lock viral surface proteins into the optimal conformation for high protective immunity, stability and manufacturing productivity, enabling the design of ready-to-use fully liquid vaccines. This technology has been invented by Prof Paul Young, Dr Daniel Watterson, Dr Keith Chappell and their groups at The University of Queensland (UQ), Brisbane, Australia. The Molecular Clamp technology has already been applied to generate vaccine candidates against several life-threatening respiratory viruses including respiratory syncytial virus (RSV), metapneumovirus, influenza and SARS-CoV-2.

UniQuest is the main commercialisation company of The University of Queensland and specialises in global technology transfer and facilitates access for all business. Notable vaccine licensing success include the blockbuster cervical cancer vaccine Gardasil®.
Publications
Development of molecular clamp stabilized hemagglutinin vaccines for Influenza A viruses.
McMillan CLD, Cheung STM, Modhiran N, Barnes J, Amarilla AA, Bielefeldt-Ohmann H, Lee LYY, Guilfoyle K, van Amerongen G, Stittelaar K, Jakob V, Lebas C, Reading P, Short KR, Young PR, Watterson D, Chappell KJ. NPJ Vaccines. 2021 Nov 8;6(1):135. doi: 10.1038/s41541-021-00395-4
Combinatorial F-G Immunogens as Nipah and Respiratory Syncytial Virus Vaccine Candidates.
Isaacs A, Cheung STM, Thakur N, Jaberolansar N, Young A, Modhiran N, Bailey D, Graham SP, Young PR, Chappell KJ, Watterson D.Viruses. 2021 Sep 28;13(10):1942. doi: 10.3390/v13101942.
Chappell KJ, Mordant FL, Li Z, Wijesundara DK, Ellenberg P, Lackenby JA, Cheung STM, Modhiran N, Avumegah MS, Henderson CL, Hoger K, Griffin P, Bennet J, Hensen L, Zhang W, Nguyen THO, Marrero-Hernandez S, Selva KJ, Chung AW, Tran MH, Tapley P, Barnes J, Reading PC, Nicholson S, Corby S, Holgate T, Wines BD, Hogarth PM, Kedzierska K, Purcell DFJ, Ranasinghe C, Subbarao K, Watterson D, Young PR, Munro TP.Lancet Infect Dis. 2021 Oct;21(10):1383-1394. doi: 10.1016/S1473-3099(21)00200-0. Epub 2021 Apr 19.
Watterson D, Wijesundara DK, Modhiran N, Mordant FL, Li Z, Avumegah MS, McMillan CL, Lackenby J, Guilfoyle K, van Amerongen G, Stittelaar K, Cheung ST, Bibby S, Daleris M, Hoger K, Gillard M, Radunz E, Jones ML, Hughes K, Hughes B, Goh J, Edwards D, Scoble J, Pearce L, Kowalczyk L, Phan T, La M, Lu L, Pham T, Zhou Q, Brockman DA, Morgan SJ, Lau C, Tran MH, Tapley P, Villalón-Letelier F, Barnes J, Young A, Jaberolansar N, Scott CA, Isaacs A, Amarilla AA, Khromykh AA, van den Brand JM, Reading PC, Ranasinghe C, Subbarao K, Munro TP, Young PR, Chappell KJ.Clin Transl Immunology. 2021 Apr 5;10(4):e1269. doi: 10.1002/cti2.1269. eCollection 2021.
Adjuvant Selection for Influenza and RSV Prefusion Subunit Vaccines.
Isaacs A, Li Z, Cheung STM, Wijesundara DK, McMillan CLD, Modhiran N, Young PR, Ranasinghe C, Watterson D, Chappell KJ.Vaccines (Basel). 2021 Jan 20;9(2):71. doi: 10.3390/vaccines9020071.
Rapid Response Subunit Vaccine Design in the Absence of Structural Information.
Wijesundara DK, Avumegah MS, Lackenby J, Modhiran N, Isaacs A, Young PR, Watterson D, Chappell KJ.Front Immunol. 2020 Nov 4;11:592370. doi: 10.3389/fimmu.2020.592370. eCollection 2020.